
You need to make a 0.5 M solution of NaCl, having decided you want 2 liters how much NaCl should you add?įirst you must calculate the number of moles in this solution, by rearranging the equation Here, the solution being used is typically defined by its molar concentration ( M). The scenario in which most lab scientists will encounter this type of calculation is when making up solutions following a standard operating procedure (SOP) or a scientific paper. Molarity ( M or mol/L) = Number of Moles (mol) / Volume (L)Įxample of molarity and concentration calculations Number of moles (mol) = Mass (g) / Molar Mass (g/mol)Ĭoncentration (g/L) = Mass (g) / Volume (L) To calculate molarity or to calculate related values (including volume, mass, molar mass and concentration) from molarity, the following equations are utilized. Provided some additional information is known, one value can be deduced from the other using the equations below. Whereas two solutions at the same concentration will have the same mass of the chemical per liter of solution but are therefore likely to have differing numbers of molecules of that chemical per liter. Two solutions that have the same molarity will have the same number of molecules of the chemical per liter but are likely to contain differing masses of that chemical per liter to achieve this. How does molarity relate to concentration? For sodium chloride (NaCl) they are in a ratio of 1:1 so the molar mass of NaCl is 22.99 + 35.45 = 58.44 g/mol.įor a compound like water (H 2O), 1 mole of hydrogen (H) is 1.008 g/mol and 1 mole of oxygen (O) is 15.9994 g/mol. One mole of sodium (Na) is 22.99 g, and 1 mole of chlorine is 35.45 g. The molar mass is the mass in grams of 1 mole of a particular molecule. So, 1 mole of hydrogen gas (H 2) contains 6.02x10 23 molecules, and 1 mole of glucose (C 6H 12O 6.) also contains 6.02x10 23 molecules, but as H 2 is a much simpler molecule, 1 mole of H 2 will have a much smaller mass (the molar mass) than 1 mole of C 6H 12O 6. This equates to roughly 6.02x10 23, also referred to as Avogadro's Number. A 1 M solution is said to be “one molar”.Ī mole is the quantity of anything that has the same number of particles as 12 g of carbon-12. Finally, calculate the percent solution using the formula: 10g/100g * 100 = 10% of the solution by mass.Molarity is the concentration of a solution in terms of the number of moles of the solute in 1 dm 3 (1 liter) of the solution.For this example, the mass of the solution is 100g. Next, determine the mass of the solution.For this example, the mass of the solute is 10g. Next, determine the mass of the solute.For this example, we will look at the percent by mass. First, determine the units you wish to use.In other words the ratio of solute to the solution of a mixture. Percent Solution DefinitionĪ percent solution is defined as the total percentage of a solution in mass or volume that a solute takes up.

To calculate the percent concentration of the solution, divide the solute mass or volume by the solution mass or volume, then multiply the result by 100.
:max_bytes(150000):strip_icc()/173637959-58befc655f9b58af5c9acab8.jpg)
Note that the solute and solution measurements must be the same unit i.e.

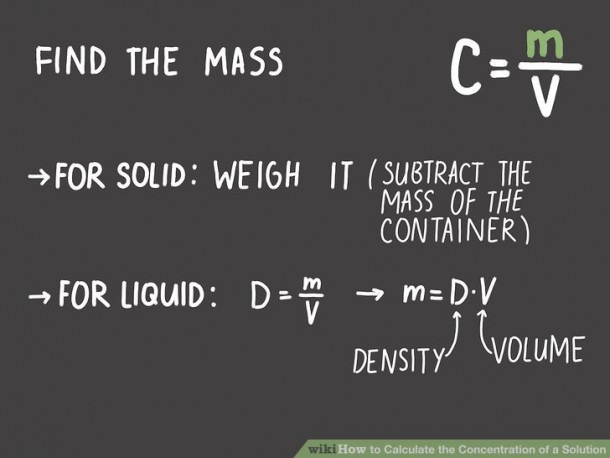


 0 kommentar(er)
0 kommentar(er)
